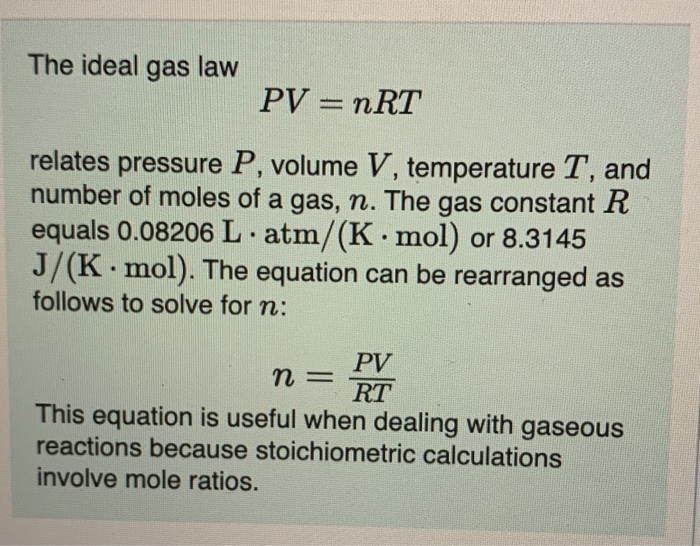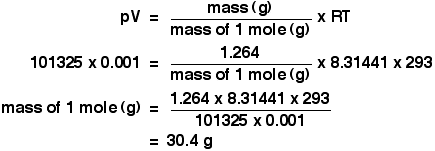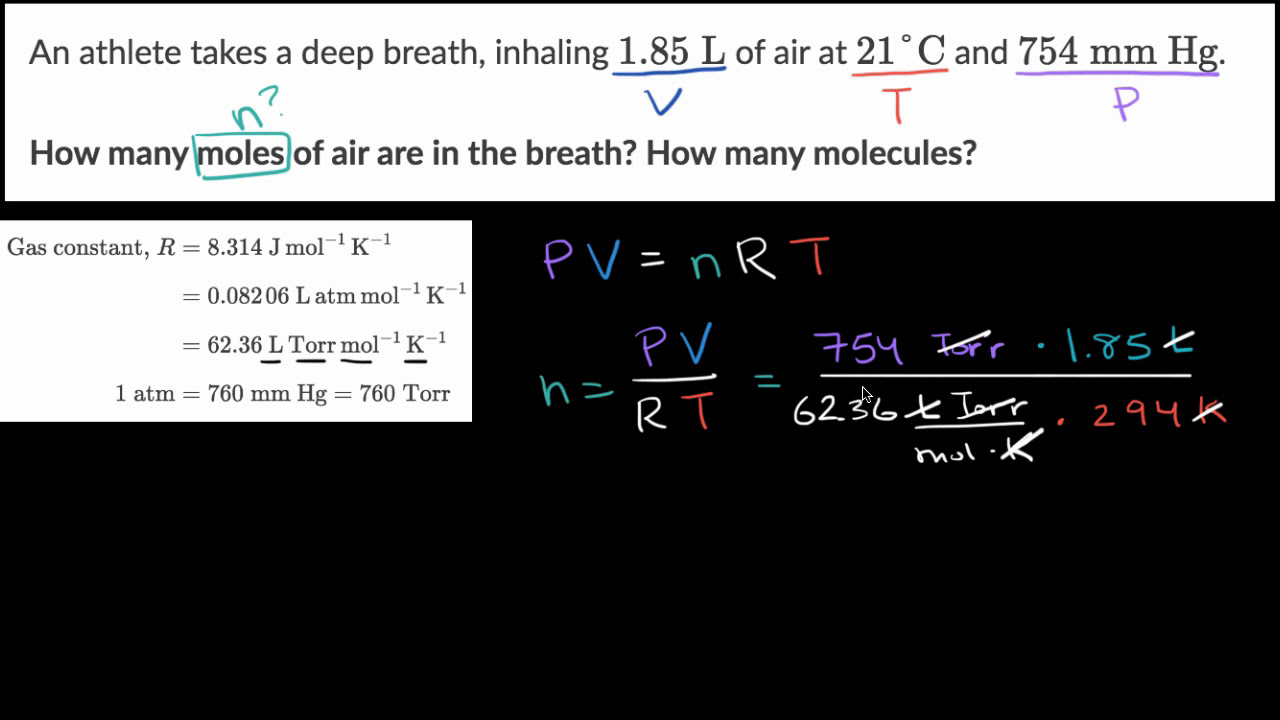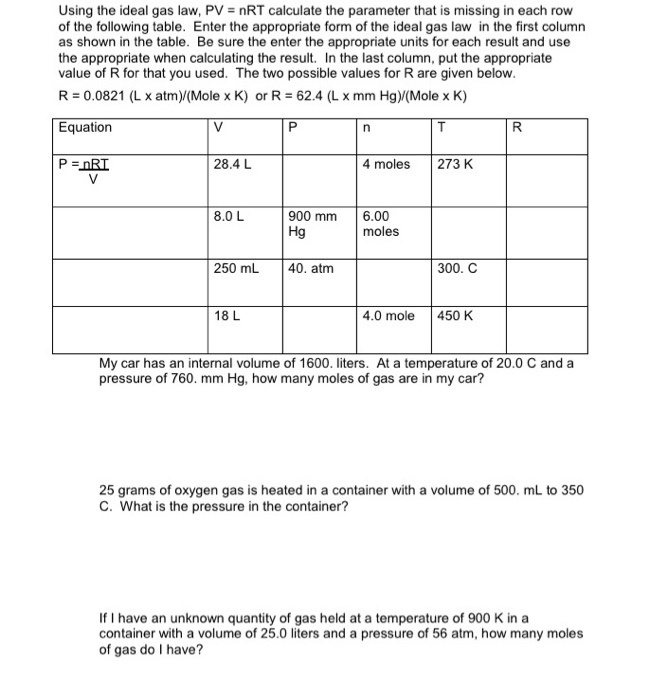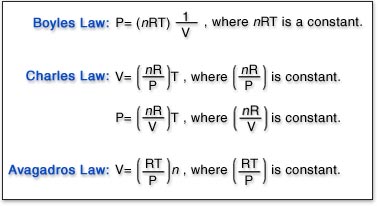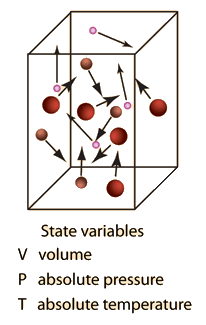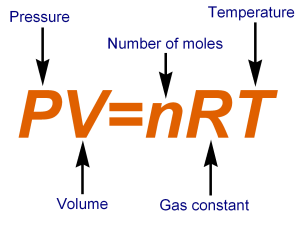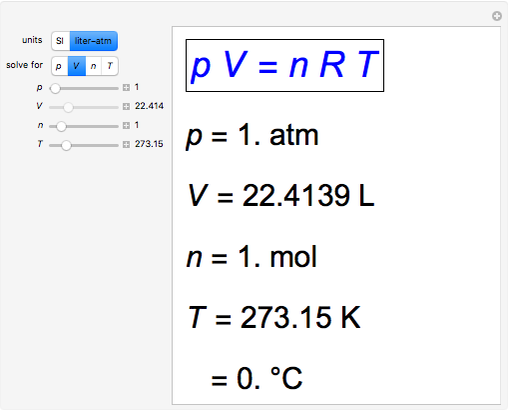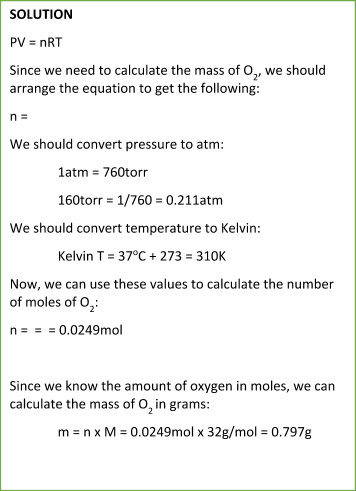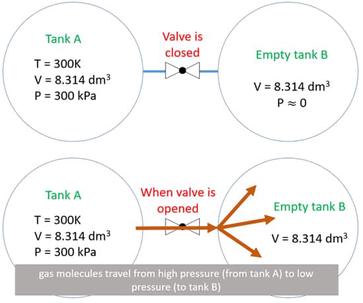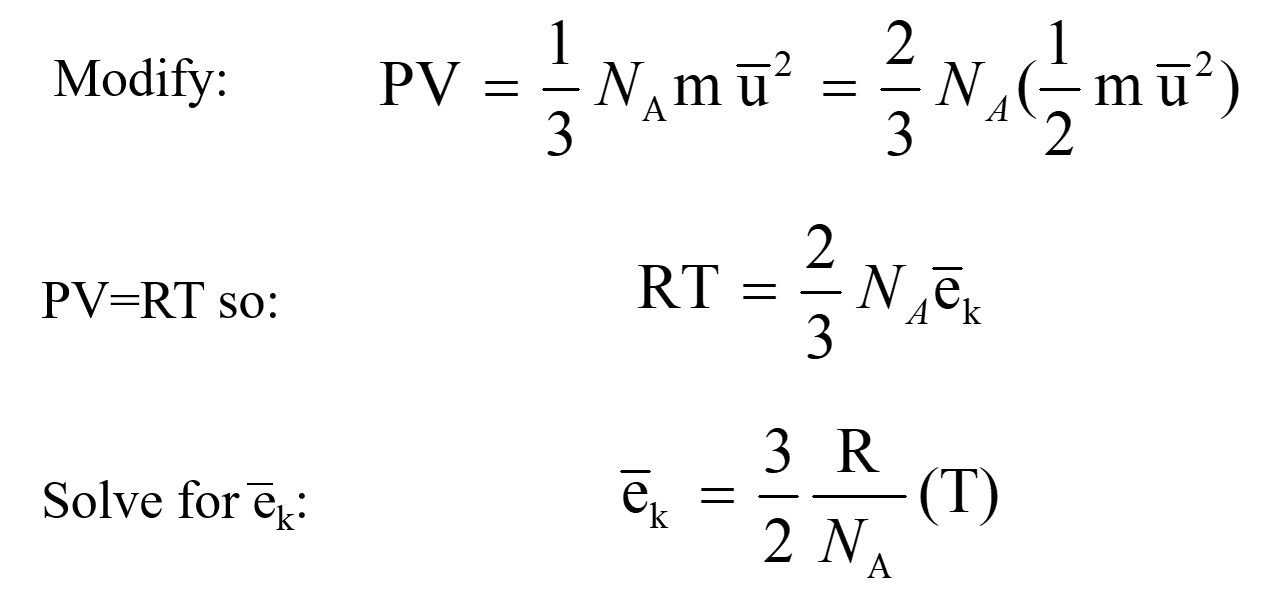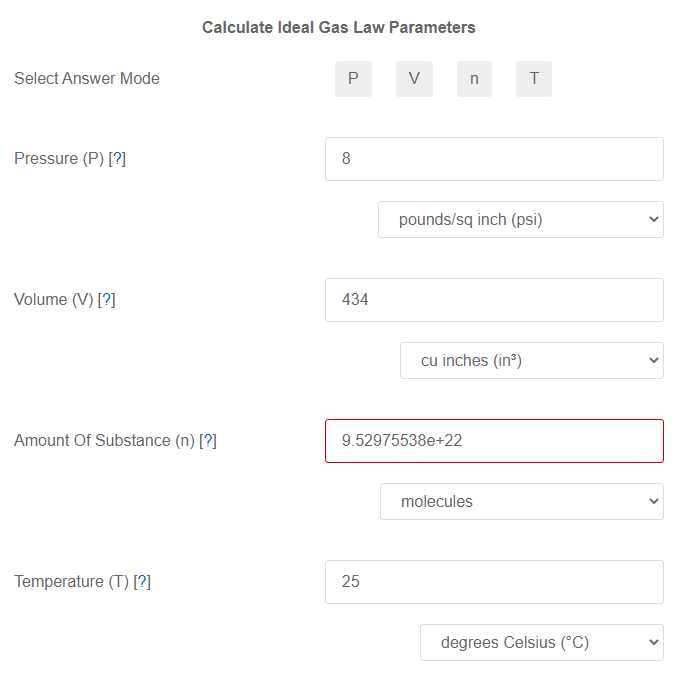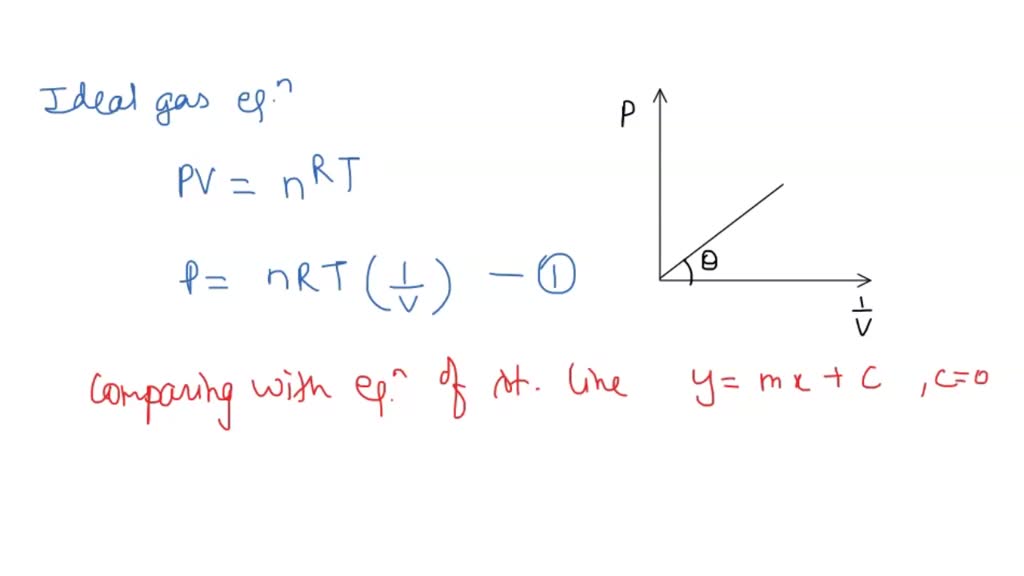
SOLVED: question In the Ideal gas equation PV = nRT How can you plot a straight line equation in P and V to find the molar gas constant; given temperature and the
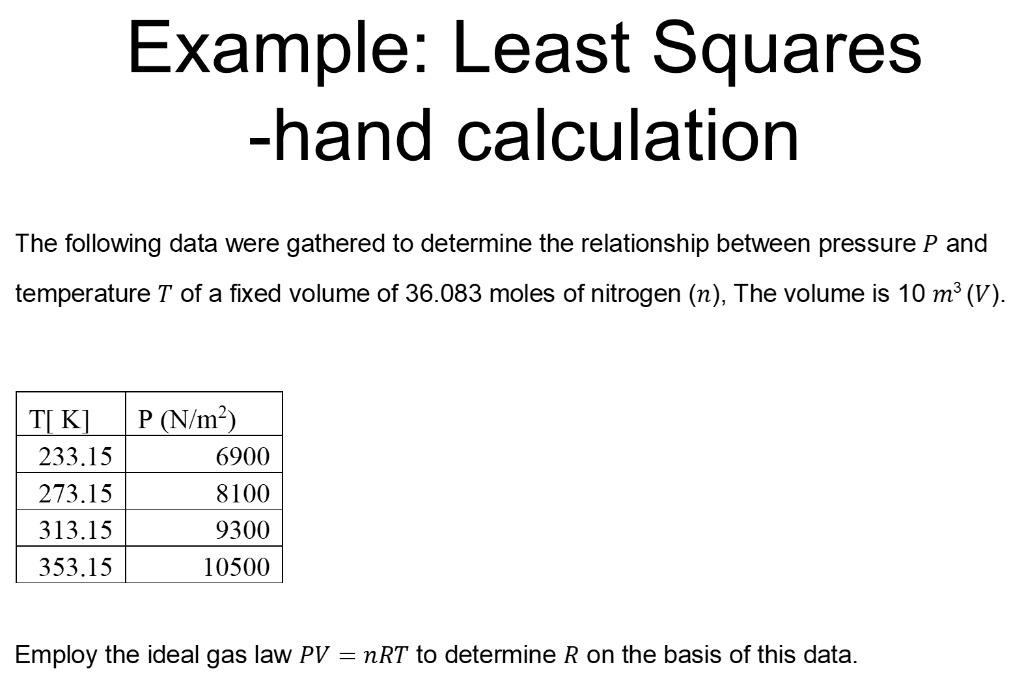
SOLVED: Example: Least Squares hand calculation The following data were gathered to determine the relationship between pressure P and temperature T of a fixed volume of 36.083 moles of nitrogen (n), The



![1.3/S1.5.1/2/4 Solve problems using the ideal gas equation, PV = nRT [SL IB Chemistry] - YouTube 1.3/S1.5.1/2/4 Solve problems using the ideal gas equation, PV = nRT [SL IB Chemistry] - YouTube](https://i.ytimg.com/vi/SEB7NA8YD9s/maxresdefault.jpg)